|
The 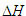 for reactions in terms of Bond Energies (bond dissociation enthalpies)...
for reactions in terms of Bond Energies (bond dissociation enthalpies)...
This segment of EXBAN presents a brief review of the 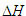 for reactions in terms of bond breaking and making events. This is done
to emphasize the large energies required to break bonds in comparison
with overall
for reactions in terms of bond breaking and making events. This is done
to emphasize the large energies required to break bonds in comparison
with overall 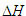 values
for reactions that can be negative or positive depending on the energy
balance of the bonds ruptured and formed. Two factors that must be kept
in mind: values
for reactions that can be negative or positive depending on the energy
balance of the bonds ruptured and formed. Two factors that must be kept
in mind:
(a) In the present depiction bonds are “pulled apart” and
the fragments “snap” together to form new bonds in “tinker-toy” fashion.
The overall 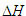 for
reactions can always be represented in this way, or in terms of the balance
of bond breaking and making events employing appropriate bond dissociation
enthalpies. The path the reaction actually takes for the transition between
reactants and products will, of course, be quite different, and depend
on the mechanism, e.g. whether the reaction is uncatalyzed, or catalyzed,
etc. for
reactions can always be represented in this way, or in terms of the balance
of bond breaking and making events employing appropriate bond dissociation
enthalpies. The path the reaction actually takes for the transition between
reactants and products will, of course, be quite different, and depend
on the mechanism, e.g. whether the reaction is uncatalyzed, or catalyzed,
etc.
(b) The misconception considered in this website is connected
with the energy associated with bond breaking. The reactions here, including
the coupled reaction, are examined, therefore, in terms of the 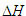 ’s
of the processes involved. Entropic contributions often contribute to
the spontaneity of reactions, and can become the principal driving force
for reactions or phase changes. It is, indeed, entropy that is responsible
for the concentration dependence of reactions. However, bond breaking
and making, as with the examples given here, is often the main factor
in determining the spontaneity of reactions. ’s
of the processes involved. Entropic contributions often contribute to
the spontaneity of reactions, and can become the principal driving force
for reactions or phase changes. It is, indeed, entropy that is responsible
for the concentration dependence of reactions. However, bond breaking
and making, as with the examples given here, is often the main factor
in determining the spontaneity of reactions.
|

